
 Tetravalency that is exhibited by carbon cannot be explained using this theory.
Tetravalency that is exhibited by carbon cannot be explained using this theory. 
This entire process can be explained using the Valence Bond Theory for coordination compounds.įollowing are certain limitations to the Valence Bond Theory for Coordination Compounds: Hence, the 1s orbital of Hydrogen(H+) overlaps with the 2pz orbital of Fluorine(F-) to form a covalent bond. In an HF molecule, both Hydrogen and Fluorine have an unpaired electron each.
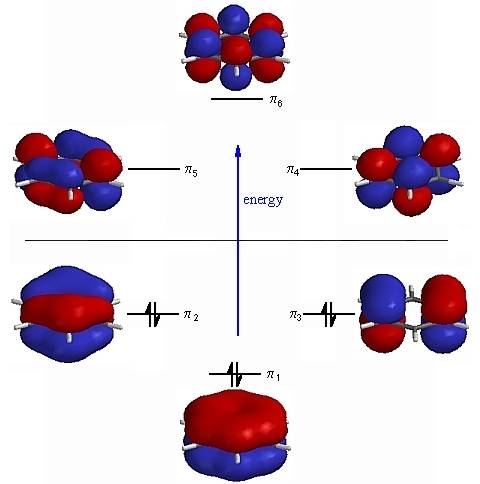
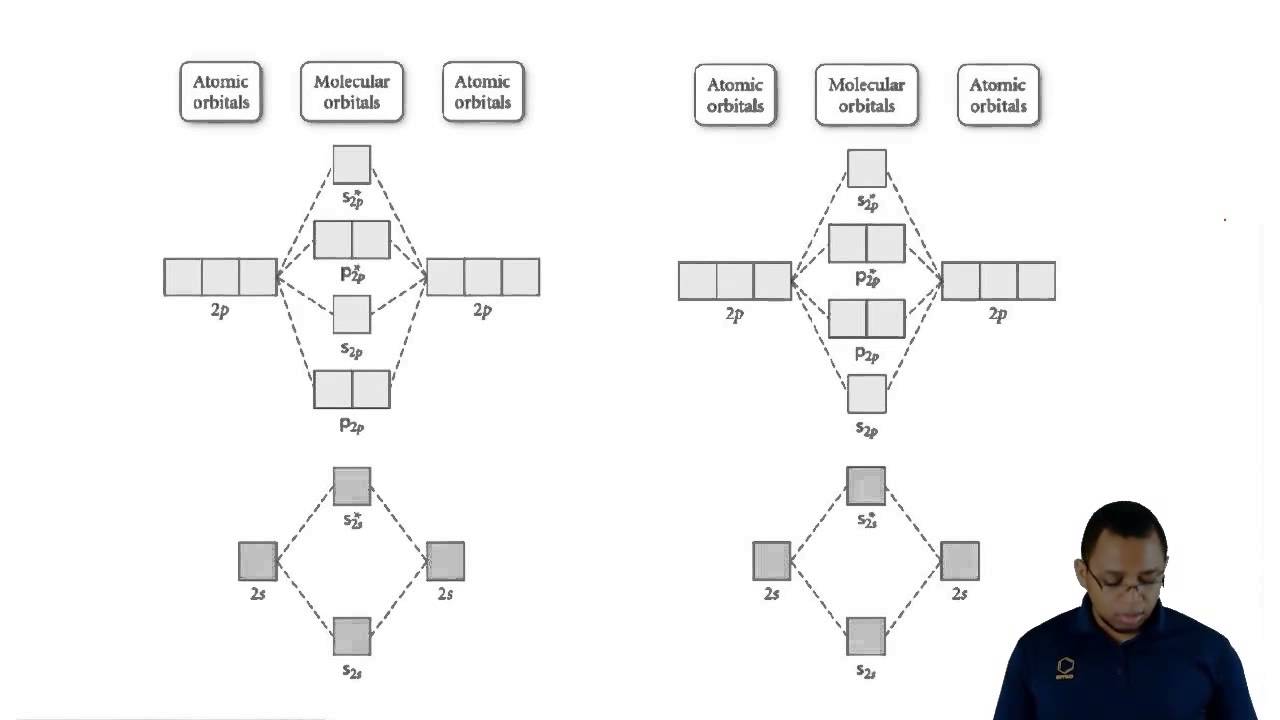 We can explain the difference in the length and strength of the chemical bonds in H2 and F2 molecules, considering the difference in their overlapping orbitals. The Valence Bond Theory for coordination compounds describes the maximum overlap condition, which explains the creation of covalent bonds in several molecules. These hybrid orbitals then overlapped with the ligand orbitals to donate electron pairs for bonding. This could also result in definite geometrical forms such as octahedral, tetrahedral, square planar, etc. The valence bond theory for coordination compounds mentions that a metal atom or ion, under the influence of ligands, can use its (n-1) d, ns, np, or ns, np, nd orbitals for hybridization to yield a set of equivalent orbitals. On the other hand, bonds formed by the overlapping of atomic orbitals along the inter nuclear axis are called Sigma bonds. Covalent bonds created by the sideways overlap of atomic orbitals are called pi bonds. Covalent bonds are directional and parallel to corresponding areas of intersecting atomic orbitals.Ĭovalent bonds can be classified into two types according to the design of the overlap, viz., a pi bond and a sigma bond. Paired electrons in the valence shell cannot participate in such a bond. Atomic orbitals possessing more than one unpaired electron can form one bond. Atoms acquire this stabilizing property as a result. The electron density between the two bonded atoms rises due to the overlap. The overlapping of two half-filled valence orbitals of two dissimilar atoms influences the formation of covalent bonds. The major postulates of the Valence Bond Theory are: The theory primarily focused on the concepts of electronic configuration, overlapping of atomic orbitals, and their hybridization. They also used Schrödinger’s wave equation to throw light on the formation of covalent bonds between two hydrogen atoms. Hence, German physicists Walter Heinrich Heitler and Fritz Wolfgang London came up with the valence bond theory to resolve both the above problems.
We can explain the difference in the length and strength of the chemical bonds in H2 and F2 molecules, considering the difference in their overlapping orbitals. The Valence Bond Theory for coordination compounds describes the maximum overlap condition, which explains the creation of covalent bonds in several molecules. These hybrid orbitals then overlapped with the ligand orbitals to donate electron pairs for bonding. This could also result in definite geometrical forms such as octahedral, tetrahedral, square planar, etc. The valence bond theory for coordination compounds mentions that a metal atom or ion, under the influence of ligands, can use its (n-1) d, ns, np, or ns, np, nd orbitals for hybridization to yield a set of equivalent orbitals. On the other hand, bonds formed by the overlapping of atomic orbitals along the inter nuclear axis are called Sigma bonds. Covalent bonds created by the sideways overlap of atomic orbitals are called pi bonds. Covalent bonds are directional and parallel to corresponding areas of intersecting atomic orbitals.Ĭovalent bonds can be classified into two types according to the design of the overlap, viz., a pi bond and a sigma bond. Paired electrons in the valence shell cannot participate in such a bond. Atomic orbitals possessing more than one unpaired electron can form one bond. Atoms acquire this stabilizing property as a result. The electron density between the two bonded atoms rises due to the overlap. The overlapping of two half-filled valence orbitals of two dissimilar atoms influences the formation of covalent bonds. The major postulates of the Valence Bond Theory are: The theory primarily focused on the concepts of electronic configuration, overlapping of atomic orbitals, and their hybridization. They also used Schrödinger’s wave equation to throw light on the formation of covalent bonds between two hydrogen atoms. Hence, German physicists Walter Heinrich Heitler and Fritz Wolfgang London came up with the valence bond theory to resolve both the above problems. /GettyImages-170075160-56a133b73df78cf772685a14.jpg)
The theory could only predict geometrically for simple combinations. On the other hand, the Valence Shell Electron Pair Repulsion (VSEPR) theory had limited application, since it could not account for the geometry of complex compounds. The Lewis approach to chemical bonding did not explain how chemical bonds are formed. The original metal bonding is covalent, and the metallic structure consists of the resonance of electron pairs between atoms and their neighbors. The atomic orbitals overlap on the bond formation, and the larger the overlap, the stronger the bond.” What does the Valence Bond Theory say?Īccording to the Valence Bond Theory, “Electrons in a molecule occupy atomic orbitals rather than molecular orbitals. It focuses mainly on the electronic configuration, orbitals, hybridization, and overlap of orbitals. Hence, the theory explains the chemical bonding of elements using quantum mechanics. We can also say that the chemical bonding of elements depends largely on the ability of atoms to share electron-pair bonds. The theory is based on the Lewis concept of the electron-pair bond. One such theory is the Valence Bond Theory. The bonding of coordination compounds has been the subject of several theories.





/GettyImages-170075160-56a133b73df78cf772685a14.jpg)


 0 kommentar(er)
0 kommentar(er)
